Battery Charger
This is actually a year-old design, I just finally got around to turning it into a nice circuit board so that I don't have to worry about heat sinks touching and shorting out. Also made it able to charge twice as many batteries as the "bunch of wires soldered in the air" version I was using before.
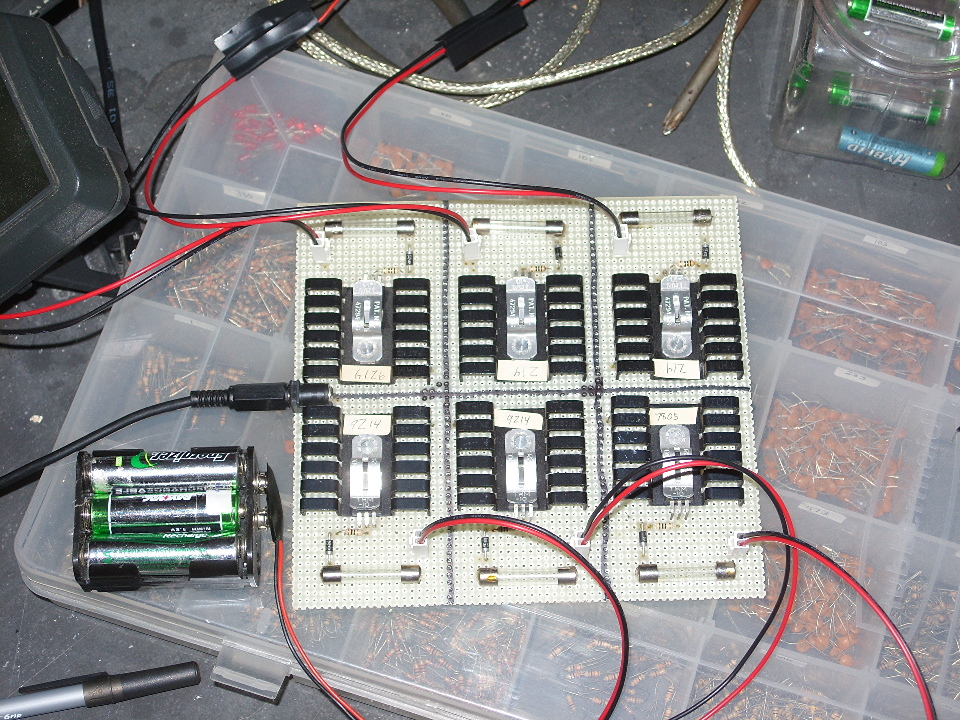
It's actually six charging circuits, each of which is simply a 125 mA current source and nothing more. Each circuit is connected to a six-cell battery holder, which means that it can charge 36 batteries simultaneously, which is useful as it takes about 24 hours to charge them.
Despite all the rage over "smart chargers," I have enough experience with battery chargers to know that "smart chargers" are dumb as fuck. "Dumb" chargers are the smart way to charge your batteries. Probably the best way to explain why is to tell the story of how I came to hold this opinion.
I used to have two "smart" chargers, each of which would charge four batteries in about an hour. Quite a lot of fun that was, since I had more than eight batteries, and NiMH batteries don't hold a charge well, so once they were charged I removed them to charge more, then by the time I got around to using them, they were dead. So I'd plop some in the charger and just pray that they'd recharge before I needed to leave to go wherever I wanted to take my camera.
Then one day one of the solder joints holding one of the temperature sensors to one of the battery terminals broke, causing the charger to fail to measure the battery's temperature. Why is this a problem? Well, normally when you overcharge a battery, it'll begin emitting gasses, causing an explosion. However, the NiMH designers were smart and included a catalyst in the mix, so that these gasses are turned back into non-gasses, and the energy that produced them turned into heat. The way the "smart" charger detects that the battery is charged is by sensing the increase in temperature when this begins to happen. Sounds almost a bit dumb, doesn't it? Needless to say, the battery became very hot, and thankfully I noticed when the odor of the burning label caused me to wonder what the fuck that smell was and investigate, and so I caught it before it caught fire.
Needless to say, I tossed that charger. I kept a close eye on the other one, and one day noticed that one of its temperature sensors had come loose too, and so I tossed it as well. Apparently it's a common failure mode, probably because the negative battery terminal is flexed every time you insert a battery, and the sensor is soldered directly to it in order to be in good thermal contact with the battery.
So, in need of a new charger, I went to the store, where I found that the one hour chargers are apparently no longer produced. Maybe they proved too dangerous. So I bought a charger that promised to charge batteries in only eight hours. Semi-smart, I guess. (Ooh, I don't have to quote "smart" this time!)
It proved to be even more useless. You had to charge the batteries in pairs, which isn't a big deal except that the charger also attempts to determine when charging is complete and stop, due to it's "smart" nature. However, two batteries are never equally discharged, and so it will always terminate charging before one of them is charged. This charger also had a terrible habit of rejecting batteries, which the manual indicated meant that they were no good and should be discarded. Sometimes I could swap them with other batteries and eventually get them to charge, other times nothing seemed to work and so I tossed about five of them, until it began giving me shit about the new batteries that came with the charger, at which point I tossed the charger.
So, off to the internet to research how to charge NiMH batteries...
After reading lots of BS about how NiMH batteries are terribly difficult to charge due to there being no significant change in voltage as they charge, I eventually came across a page with some useful information:
http://www.talkingelectronics.com/projects/ChargingNiMH/ChargingNiMH.htmlI read that page for a while before realizing I had stumbled upon anything useful, as it was mostly talking about trickle chargers which I wasn't that interested in, being "smart" charger-obsessed like most people. I wasn't clued in to how awesome "dumb" chargers are until I read this:
Believe it or not, you can mix any size and type of cell with this circuit as each will become fully charged after a period of 2 days or more, without you having to think about the situation. You can mix any type of cells (NiCad, Li-ION, NiMH) and any size (AA, C or D) and they will gradually charge according their own characteristics and capacity. You don't have to worry about anything.
As it turns out, all of that concern about over-charging batteries is only an issue because of the "smart" chargers. The batteries contain a catalyst which transforms any gassing due to overcharging back into the battery's normal chemistry, releasing the excess energy as heat. Under slow charge conditions, this is fine, because the battery only becomes a little warm. However, if you insist on charging your battery in only an hour, then you'll be pushing so much energy into the battery that if you don't detect when it is fully charged and stop pushing more energy into it, you'll catch something on fire if the battery doesn't just explode.
So, there's two solutions to that problem:
1. Design a smart charger with some well thought out but poorly performing method of determining when the battery is charged, which will necessarily sometimes fail to fully charge the battery, and other times overcharge it, and toss in a temperature sensor to detect when you're wrong so that you can stop over-charging before a fire erupts. As for the undercharging, eh, just pretend it doesn't happen.
2. Just don't charge the battery so fucking quickly.
Honestly, the concern about over-charging is nothing more than that. I've been over-charging my batteries for a year by leaving them on my charger all the fucking time when I'm not using them. They're just fine. They don't explode, swell, leak, or even lose capacity. They just get a little warm (about body temperature) and stay that way.
Anyway, the interesting thing about over-charging is that the battery continues to conduct current as it over-charges. This means that if it is wired in series with other batteries that aren't fully charged yet, they'll continue to charge until they too are over-charging. This is quite useful since it allows you to charge more batteries with less circuitry. Also, by over-charging, you're sure that your batteries are charged, vs. using a "smart" charger which is going to stop charging because it has erroneously determined that the battery is fully charged.
Again, I've been over-charging my batteries for a year. There appears to be no ill effects of doing so, despite the many web pages out there that insist it will reduce their lifespan. The data from my battery tester below fails to show any damage from overcharging, and knowing how it works (the catalyst and all) it isn't obvious why it should be a problem assuming the rate of overcharging is small.
Anyway, the circuit I've been using is the last one on the page linked above, under the heading "How the Circuits Work." I have no idea why that guy is using a transistor in the other schematics, as the LM317 and resistor alone seem to do the job just fine. I've been using a 10 ohm resistor, for a current output of 125 mA. I also added a 300 mA fuse just in case the regulator fails, and a diode to prevent discharge if the charger's power source is removed, which I discovered causes enough reverse current to burn up the resistor. Never bothered to figure out the exact time it takes to charge fully-dead batteries, as it's just never been an issue, but I suspect it's about a day.
Turns out that if you have enough battery chargers, you just keep all your batteries in a charger all the time, and so your need to charge batteries quickly quickly disappears. (I love when I get to use the same word twice consecutively. I usually only get to do that with "that," e.g. "I know that that is weird.") When you need batteries, you just pick some out of the charger and use them, and by simply being sure to always pick the pack which has been in the charger the longest, they've usually always been in the thing for at least a week before I use them, so they're certainly charged despite the long charge time.
I initially planned to redesign this circuit to switch to an even lower current charge after some time (by simply pulsing the initial current at a 10% duty cycle), either after a set time period (like 48 hours) or by detecting when the batteries become fully charged. One web page recommended charging to 1.4 volts/cell, then switching off the charge until it falls back down to 1.3 volts/cell. It seemed like a wonderfully simple idea, but a simple test of discharging some cells and placing them on my charger showed that will leave cells undercharged. You really need that period of overcharging in order to ensure that even the weakest cells receive a full charge. I might still consider the timer method since it won't have that problem, but given the added complexity of putting a microcontroller into the otherwise simple charging circuit, it's hard to be motivated to do that when testing with my battery discharger fails to show any negative effects from the overcharging I've been giving my batteries over the last year.
Battery Discharger
Last year I decided I needed a way to test the capacity of my rechargeable batteries, after that PITA semi-smart charger started rejecting so many batteries. Not sure how it led to that. I remember using a single-cell holder to completely discharge batteries for some reason. Maybe it was making it more likely to accept them? I dunno. Then I thought I should automate this and measure the voltage as it discharges. Yeah, I'm not sure what I was hoping to accomplish, but it led to a good idea anyway.
It seemed easy enough to measure the capacity of the batteries. Just fully charge them, then short the batteries with a small resistance and monitor voltage until they go dead. I ordered some parts, even put little number labels on my batteries so that I could tell them apart, but never built the thing. At least, not until last week:
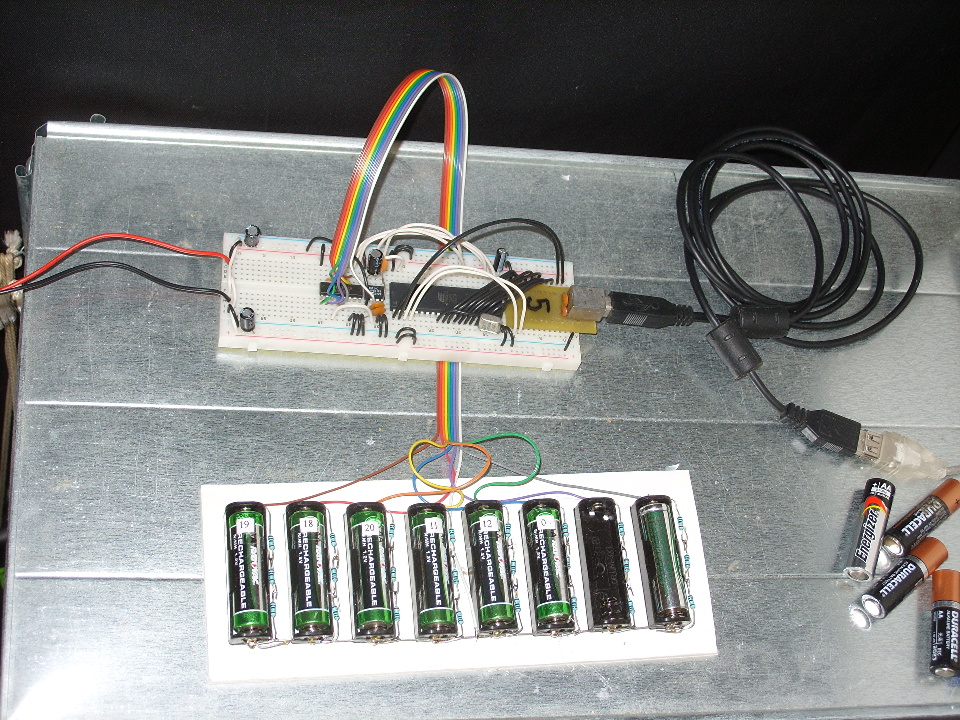
Each cell is connected to three 1 ohm resistors in series. The resistors are 1% tolerance, the first time I've ever bothered to use anything more accurate than the usual. The batteries are then all connected to a 74HC4051, an eight-channel analog multiplexer, which switches which battery is connected to the ADC which measures the voltage. Measurements are taken once per second and sent to the PC, which records the data and makes pretty graphs:
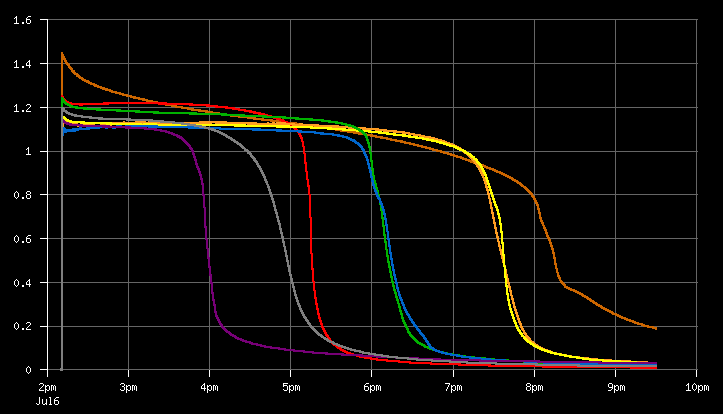
This is from the first batch I tested. The brown line is an alkaline Duracell that I've been carrying around in my pocket for a year. (just curious if it was still good-as-new like Duracell claims) All of the others are NiMH cells which were fully charged, maybe, really I didn't care since I was just testing the device, but I have tested all of my batteries after a full charge since then and this result looks totally typical.
You can see how the alkaline battery actually decreases in voltage as it discharges, but the NiMH voltages remain relatively steady.
Anyway, the useful thing to do with this data is to calculate the current since we know the voltage and the resistance, then with that calculate the power, and with that, calculate how much total energy is in the battery.
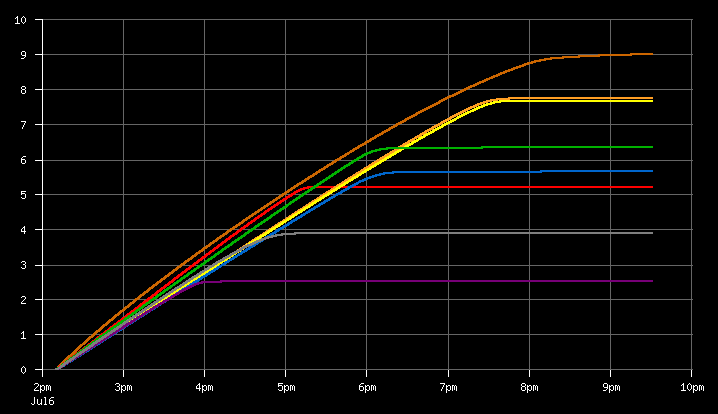
The graph is labeled in kilojoules. The time axis is essentially irrelevant, with only the final level on the right side being important. This really should just be a bar graph but I'm too lazy to read the Ploticus manual anymore.
I built this thing so that I can pair my batteries according to how long they'll last. If I pair together a battery with 10 kJ and another with 5 kJ and put them in something, one of two things is going to happen:
1. When the 5 kJ goes dead, the device detects a low voltage and stops working, and so I never get to use the other half of the 10 kJ battery.
2. The device doesn't detect the low voltage and continues to work, which eventually puts a negative charge on the 5 kJ battery, which is bad for its chemistry.
On the other hand, if I pair that 5 kJ battery with another 5 kJ battery, it's still perfectly fine for anything I don't care running for only half as long. I hate to change batteries in my camera, but a flashlight? Who cares, just pull out the old ones and toss in new ones, plus the thing will put out a weaker light essentially forever, so it isn't like it dies without warning like the camera does. So there's no need to throw the things away, I just need to know which are which so that I can use them accordingly.
What I found is that the batteries performance is essentially related to age, but not as strongly as I initially expected. The oldest batteries, at five years old, range from 4.2 to 5.7 kJ, with one outlier at 3.5 kJ which I probably will throw away since it's so far out of line that I can't pair it with anything.
The most interesting thing I found was that the year-old batteries I got with that terrible charger are the best performing batteries I have, even compared to the new ones I bought two weeks ago and have never used. The year-old batteries scored between 9.4 kJ 9.6 kJ, whereas the new ones test between 6.1 and 6.6 kJ.
The difference seems to be that the new ones are the "low self-discharge" type, which other sources on the internet tell me are usually about 2/3 the capacity of the original type, which will self-discharge in about a month if not left on a charger. However, given my method of use, which involves leaving them on the charger all the fucking time, I guess I need to find more of the original variety.
As for the harm of constant overcharging, I do have more of the variety I bought last week, which I bought at least a year ago and have been constantly overcharging for the last year. They test between 6.5 and 7.0 kJ. Yes, that's right, they're better than the new ones. I don't know why that is, but it certainly doesn't point to overcharging being an issue with battery life.
As for the more poorly performing five year old batteries, I suspect most of their damage came from the "smart" chargers I used to charge them. The things were so fucking hot by the time charging completed that I always suspected they were being damaged a little bit each time.
My new charger, in it's slow-charging awesomeness, doesn't make the batteries very warm at all. You can definitely feel the warmth, especially if you compare the feel of some fully charged batteries to some which were completely dead and have been charging for only 12 hours, as they'll be cold and the charged ones will be warm, but without that comparison it might be easy to mistake them for not being warm as they seem just a little above body temperature.



